Table Of Contents
Central Drugs Standard Control Organisation Registration
CDSCO registration is a mandatory process for all manufacturers, suppliers, and distributors of drugs. The registration process helps in maintaining transparency in the drug supply chain and combating counterfeiting. It also helps in registering bulk drugs from various sources.
CDSCO has set up a database that contains information about all registered CDSCO-registered manufacturers, suppliers, and distributors of bulk drugs in India. The database is accessible to authorized officers of the Directorate General of Health Services (DGHS), Ministry of Health & Family Welfare.
The following applicants can register on CDSCO online registration portal:
- Importers
- Foreign Enterprises holding Indian Subsidiary
- Corporates
- Indian Agents
Registration Steps For CDSCO Online Registration
- Open link”www.cdscoonline.gov.in” and then click on”Sign Up Here” (highlighted) to register yourself,
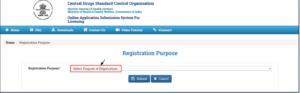
- After clicking on the “Sign Up Here” link for “Registration Purpose” on the portal,a new window will open
- After clicking on the drop-down, the list for the Purpose of Registration is open
- User can choose any of the registration purposes

- After selecting the Registration Purpose, the user has to submit by click on the “Submit” button.
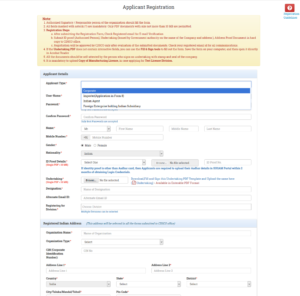
- Then after fill the Registration Form and submit
- After getting the Successful Registration message,a confirmation link will be sent to the user’s registered email id to verify registration as shown in Figure 1.15
- User can activate accounts by clicking on the link sent to the registered email id.
- When the user will click on the verification link sent to the user’s registered email id , application will send for approval to the concerned authority(CDSCO Officials)
List of new drugs approved in the year 2022 till date
Here are the new drugs approved list
CDSCO Online Registration Guidelines
Central Drugs Standard Control Organisation (CDSCO) has issued a new set of online registration guidelines for online registration. CDSCO is an agency under the Ministry of Health and Family Welfare, Government of India. It regulates pharmaceuticals, cosmetics, medical devices, and other products through a centralized system.
The new guidelines have been issued to make it easy for manufacturers to register their products with CDSCO using the prescribed form. The guidelines also make it mandatory for manufacturers to submit the required documents along with their product samples during initial registration. DST Registration Number is mandatory to fill the Formulation R&D Registration Form.
Drug of details:
Following drug details need to fill by the applicant as shown below:
- Category of Drug
- Gene Name
- Pharmacopeial monograph
- Class of Drug
- Shelf Life
Read also: 7 Steps To Improve Personal Development Skills